Choose seasonal influenza prevention across generations
APPROVED FOR PATIENTS6+ MONTHS1
A seasonal influenza vaccine for a wide range of ages
Order TodayStand strong against influenza with AFLURIA QUADRIVALENT
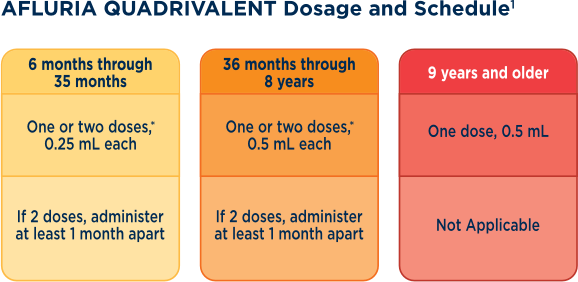
- Administer as a 0.25-mL dose for patients 6 through 35 months, and as a 0.5-mL dose for patients 36 months through 8 years as 1 or 2* doses1
- Demonstrated immunogenicity in children and adults1
- Demonstrated safety profile1
- Produced through traditional egg-based manufacturing1
*1 or 2 doses depends on vaccination history as per Advisory Committee on Immunization Practices annual recommendations on prevention and control of influenza with vaccines.
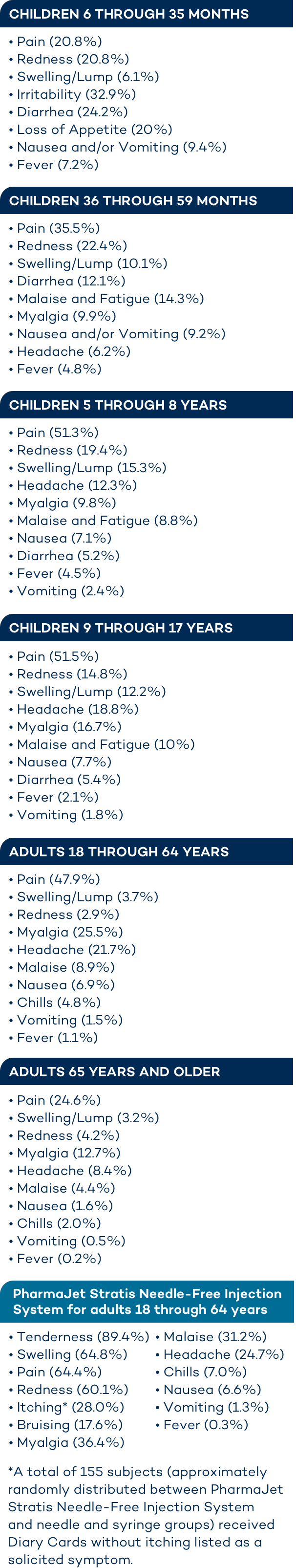
Demonstrated safety profile of AFLURIA QUADRIVALENT1
APPROVED FOR PERSONS 6 MONTHS OF AGE AND OLDER.1
Proportion of subjects per age with any solicited local adverse reactions or systemic adverse effects within 7 days after administration of AFLURIA QUADRIVALENT.1
The safety and efficacy of AFLURIA QUADRIVALENT in persons less than 6 months of age have not been established.1
The PharmaJet® Stratis® Needle-Free Injection System is not approved as a method of administering AFLURIA QUADRIVALENT to persons less than 18 and greater than 64 years of age due to lack of adequate data supporting safety and efficacy in these populations.
For intramuscular injection only, by needle and syringe (6 months and older) or by PharmaJet® Stratis® Needle-Free Injection System (18 through 64 years).1
AFLURIA QUADRIVALENT is supplied in 2 product presentations: as a package of ten 0.5-mL single-dose, prefilled, needleless syringes, or as a 5-mL multidose vial.1
Administer as a 0.25-mL dose for patients 6 through 35 months, and as a 0.5-mL dose for patients 36 months through 8 years as 1 or 2* doses.1
Both the multidose vial and prefilled syringe presentations are not made with natural rubber latex.1
*1 or 2 doses depends on vaccination history as per Advisory Committee on Immunization Practices annual recommendations on prevention and control of influenza with vaccines.
Store AFLURIA QUADRIVALENT refrigerated at 2°C to 8°C (36°F to 46°F). Protect from light. Do not freeze. Discard if the vaccine has been frozen. Do not use it after expiration date.1
Choose AFLURIA QUADRIVALENT for your eligible patients 6+ months1
Order NowAccess Featured Resources
Influenza impacts millions of people in the US4
Learn about the burden of influenza
INDICATION AND IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
Do not administer FLUAD QUADRIVALENT or AFLURIA QUADRIVALENT to anyone with a history of severe allergic reaction (e.g. anaphylaxis) to any component of the vaccine, including egg protein, or to a previous influenza vaccine.
Do not administer FLUCELVAX QUADRIVALENT to anyone with a history of severe allergic reactions (e.g. anaphylaxis) to any component of the vaccine.
WARNINGS AND PRECAUTIONS
If Guillain-Barré syndrome (GBS) has occurred within 6 weeks of receipt of prior influenza vaccine, the decision to give FLUAD QUADRIVALENT, FLUCELVAX QUADRIVALENT, or AFLURIA QUADRIVALENT should be based on careful consideration of the potential benefits and risks.
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of the vaccine.
Syncope (fainting) may occur in association with administration of injectable vaccines. Syncope can be accompanied by transient neurological signs such as visual disturbance, paresthesia, and tonic-clonic limb movements. Ensure procedures are in place to avoid falling injury and to restore cerebral perfusion following syncope by maintaining a supine or Trendelenburg position.
The immune response to FLUAD QUADRIVALENT, FLUCELVAX QUADRIVALENT, or AFLURIA QUADRIVALENT in immunocompromised persons, including individuals receiving immunosuppressive therapy, may be lower than in immunocompetent individuals.
Vaccination with FLUAD QUADRIVALENT, FLUCELVAX QUADRIVALENT, or AFLURIA QUADRIVALENT may not protect all vaccine recipients against influenza disease.
ADVERSE REACTIONS
FLUAD QUADRIVALENT:
The most common (≥ 10%) local and systemic reactions with FLUAD QUADRIVALENT in elderly subjects 65 years of age and older were injection site pain (16.3%), headache (10.8%) and fatigue (10.5%). Other adverse events may occur.
FLUCELVAX QUADRIVALENT:
In children 6 months through 3 years of age who received FLUCELVAX QUADRIVALENT, the most commonly reported injection-site adverse reactions were tenderness (27.9%), erythema (25.8%), induration (17.3%) and ecchymosis (10.7%). The most common systemic adverse reactions were irritability (27.9%), sleepiness (26.9%), diarrhea (17.9%) and change of eating habits (17.4%).
In children 2 through 8 years of age who received FLUCELVAX QUADRIVALENT, the most commonly reported injection-site adverse reactions were tenderness (28.7%), pain (27.9%) and erythema (21.3%), induration (14.9%) and ecchymosis (10.0%). The most common systemic adverse reactions were sleepiness (14.9%), headache (13.8%), fatigue (13.8%), irritability (13.8%) and loss of appetite (10.6%).
In children and adolescents 9 through 17 years of age who received FLUCELVAX QUADRIVALENT, the most commonly reported injection-site adverse reactions were injection site pain (21.7%), erythema (17.2%) and induration (10.5%). The most common systemic adverse reactions were headache (18.1%) and fatigue (17.0%).
In adults 18 through 64 years of age who received FLUCELVAX QUADRIVALENT, the most commonly reported injection-site adverse reactions were pain (45.4%), erythema (13.4%) and induration (11.6%). The most common systemic adverse reactions were headache (18.7%), fatigue (17.8%) and myalgia (15.4%).
In adults ≥65 years of age who received FLUCELVAX QUADRIVALENT, the most commonly reported injection-site adverse reactions were pain (21.6%) and erythema (11.9%).
Other adverse events may occur.
AFLURIA QUADRIVALENT:
AFLURIA QUADRIVALENT administered by needle and syringe:
In children 6 months through 35 months of age, the most frequently reported injection site reactions in the clinical study with AFLURIA QUADRIVALENT administered by needle and syringe were pain and redness (≥ 20%). The most common systemic adverse events were irritability (≥ 30%), diarrhea and loss of appetite (≥ 20%).
In children 36 through 59 months of age, the most commonly reported injection site reactions in the clinical study with AFLURIA QUADRIVALENT administered by needle and syringe were pain (≥ 30%) and redness (≥ 20%). The most commonly reported systemic adverse events were malaise and fatigue, and diarrhea (≥ 10%).
In children 5 through 8 years, the most commonly reported injection-site adverse reactions when AFLURIA QUADRIVALENT was administered by needle and syringe were pain (≥ 50%), redness and swelling (≥ 10%). The most common systemic adverse event was headache (≥ 10%).
In children 9 through 17 years, the most commonly reported injection-site adverse reactions when AFLURIA QUADRIVALENT was administered by needle and syringe were pain (≥ 50%), redness and swelling (≥ 10%). The most common systemic adverse events were headache, myalgia, and malaise and fatigue (≥ 10%).
In adults 18 through 64 years of age, the most commonly reported injection-site adverse reaction observed in clinical studies with AFLURIA QUADRIVALENT administered by needle and syringe was pain (≥ 40%). The most common systemic adverse events observed were myalgia and headache (≥ 20%).
In adults 65 years of age and older, the most commonly reported injection-site adverse reaction observed in clinical studies with AFLURIA QUADRIVALENT administered by needle and syringe was pain (≥ 20%). The most common systemic adverse event observed was myalgia (≥ 10%).
AFLURIA (trivalent formulation) administered by PharmaJet Stratis Needle-Free Injection System:
The safety experience with AFLURIA (trivalent formulation) is relevant to AFLURIA QUADRIVALENT because both vaccines are manufactured using the same process and have overlapping compositions.
In adults 18 through 64 years of age, the most commonly reported injection-site adverse reactions observed in a clinical study with AFLURIA (trivalent formulation) using the PharmaJet Stratis Needle-Free Injection System were tenderness (≥ 80%), swelling, pain, redness (≥ 60%), itching (≥ 20%) and bruising (≥ 10%). The most common systemic adverse events were myalgia, malaise (≥ 30%) and headache (≥ 20%).
Other adverse events may occur.
To report SUSPECTED ADVERSE REACTIONS, contact CSL Seqirus at 1-855-358-8966 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
Before administration, please see the full US Prescribing Information for FLUAD QUADRIVALENT, FLUCELVAX QUADRIVALENT and AFLURIA QUADRIVALENT.
FLUAD® QUADRIVALENT, FLUCELVAX® QUADRIVALENT, AFLURIA® and AFLURIA® QUADRIVALENT are registered trademarks of Seqirus UK Limited or its affiliates.
PharmaJet® and STRATIS® are registered trademarks of PharmaJet.
References:
1. AFLURIA QUADRIVALENT. Package insert. Seqirus Inc. 2022. 2. Medicare.gov. Flu shots. Accessed February 5, 2023. https://www.medicare.gov/coverage/flu-shots 3. Centeres for Disease Control and Prevention. Information for the 2023-2024 flu season. Accessed February 5, 2023. https://www.cdc.gov/flu/season/faq-flu-season-2023-2024.htm